Project details: Our previous studies have shown that variations in the endogenous ROS levels represent one of the long-sought after, early-life stochastic factors that individualize healthspan and lifespan. We now seek to understand how this transient presence of ROS exerts its long-term effects. Specifically, we investigate the extent to which changes in the redox-sensitive epigenome during development affect physiology later in life and identify target genes whose altered expression is ultimately responsible for the observed benefits at the organismal level. Our goal is to provide insights into this new regulatory role of ROS as a source for inter-individual variability and a pro-health signal, with the potential to identify late-stage interventions that promote lifespan and/or protect against age-related pathologies. For this purpose, we use advanced genetic and optogenetic techniques to manipulate with spatiotemporal resolution ROS production and the expression of key redox sensitive targets as well as “omics” approaches to globally identify new redox sensitive pathways.

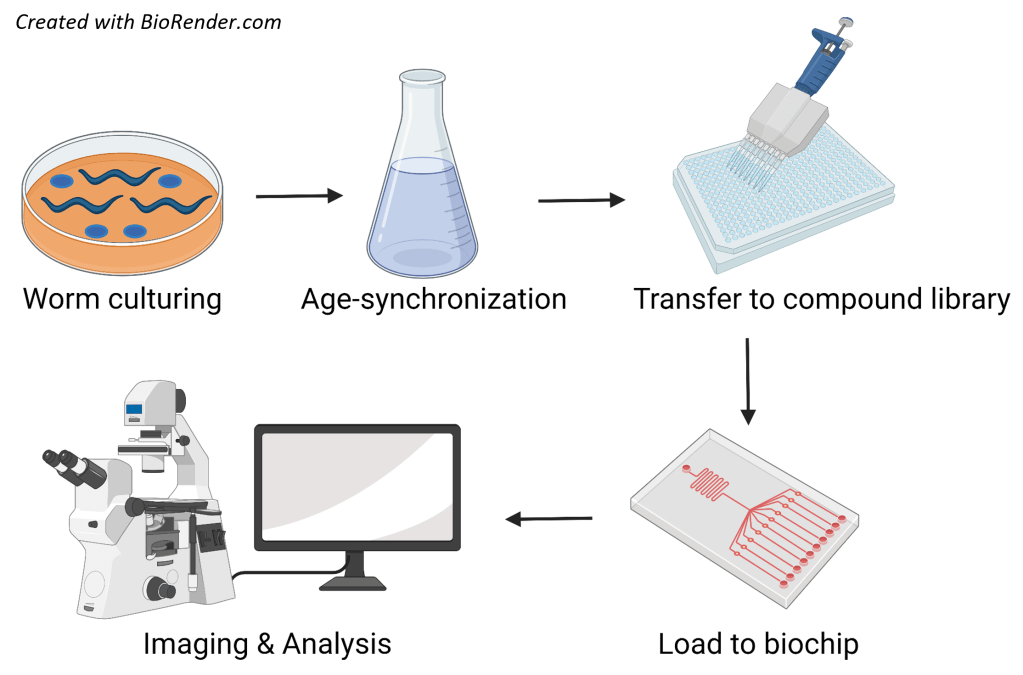
Project details: We use microfluidic-based platforms for a) high-throughput functional imaging of neurons and b) high resolution, whole-organism phenotyping. In the past, this technology allowed us to characterize the age-dependent, functional decline of C. elegans neurons and to perform a screen for the identification of compounds that preserve neuronal function with age. Moreover, we were able to monitor the onset and patterns of the disease-causing, α-synuclein inclusions in a C. elegans model of Parkinson’s disease and make associations with aspects of its physiology. We now exploit this technology to identify neuroprotective compounds with translational potential and disease-associated markers using C. elegans models of neurodegeneration.
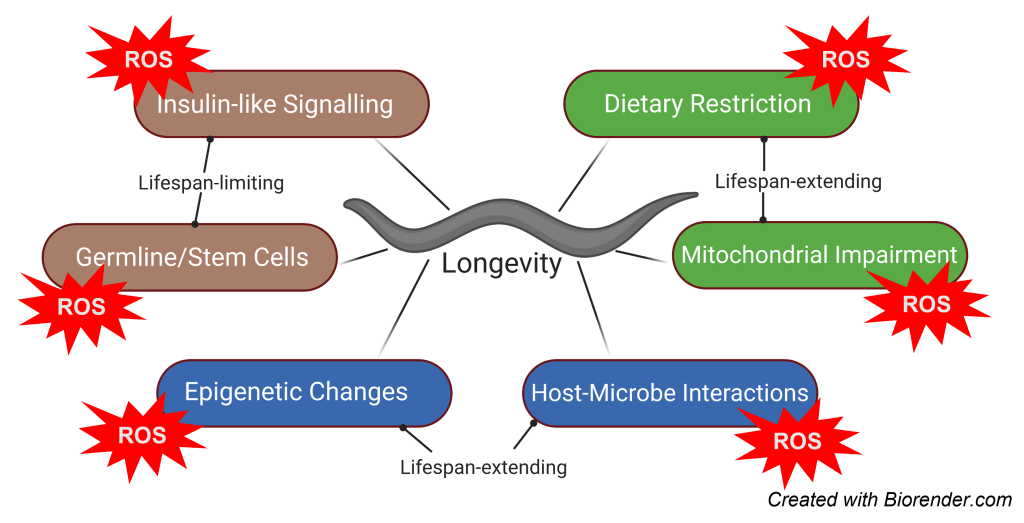
Project details: C. elegans is an invaluable model system for studies on aging, age-related diseases, and mechanisms of longevity. Lifespan-increasing mutations (e.g., mutation in daf-2 which disrupts the Insulin/IGF-1 signaling pathway) and regiments (e.g., dietary restriction, metformin) were found to impinge molecular signals which also shaped our understanding of mammalian aging. Moreover, ROS production (at low doses since higher doses are unquestionably detrimental) has shown to increase healthspan and lifespan via adaptive responses that involve improved defense mechanisms and stress resistance. However, whether known longevity modulators act independently of ROS or interact to regulate organismal lifespan, is yet unclear. To address this, we study 1) the role of ROS as a universally relevant signal for lifespan determination and 2) the implication of redox-regulated events as a downstream effectors of longevity interventions.
Funding

TWIN4EarLiStAge (HORIZON-WIDERA-2023-ACCESS-02) 2024-2027 (Coordinator)

Φυτώριο Ιδεών – Ideas Incubator 2024 (PI)

Technology Transfer and Innovation Hub Grant 2024 (PI)
Research Grant (Special Account for Research Funds of University of Crete) 2023-2025 (PI)

Flagship Action 2023-2025 (Partner)

Biomedical Research Grant 2022-2024 (PI)